Standard Medical Grade Power Products That Are TREPP Optimally Designed

Best known for our strong legacy in medical applications for powering ventilators and infusion pumps, SL POWER is here to support the shortage of medical equipment we are experiencing worldwide. Power supplies for medical and healthcare equipment have to meet not only the functional parameters of your clinical application but also safety approvals such as IEC 60601-1-2 and the 4th edition standards. This standard defines the basic and essential performance for medical equipment in regard to emissions and immunity to electromagnetic disturbances that help power medical equipment to include home use healthcare.
We have built a portfolio of standard medical grade Power Products that are TREPP optimally designed and outperform the most state-of-the-art products within their class ensuring seamless integration with the end equipment. Our goal is to reduce the time to market, which saves on development costs and opportunity loss.
During this COVID-19 emergency, we are seeing a shortage of medical equipment, and to combat this, we are staying true to our mission of saving and sustaining lives by proactively allocating resources to support the global need.

Fig.1
•Global medical device OEM experience, with broad domain knowledge in North America, Europe, and Asia.
•Rapid responses time to RFQ
•Rapidly customize to meet diverse medical device OEM needs
•Dual manufacturing locations - Mexico and China are operational 100%
•Rapid supply chain reaction time, with active raw material component stock at supporting partners
•Commercial and regulatory support to get rapid FDA approval
TREPP is not only an acronym representing the five performance dimensions of power conversion but a standard that SL Power's engineers strictly adhere to. Optimizing TREPP is a primary consideration throughout the entire product development cycle and a driving force that guides engineering decisions.
We also have the processes and experience needed by medical equipment makers. Our proprietary customer-centric product development process identifies and mitigates design, engineering, manufacturing, and sourcing risk through industry-standard (DFMEA, PFMEA, ISO 14971) and proprietary tools.
In-house evaluation and testing laboratories minimize expensive certification costs, ensure rapid development cycles and significantly improve the operational reliability of our power supplies.
We Power Medical Applications That Matter
SL Power medical power supplies can be found in virtually every healthcare application category, including surgical technologies, patient monitoring, and home care applications. In addition to power supplies that meet the Type B classification, we are one of just a handful of companies that offers Type BF and Type CF rated power supplies. These classifications are part of the 'applied parts' context of the IEC 60601 specification and relate to parts of a medical device that may come into physical contact with the patient during its normal operation.
Type B (body) is the least stringent classification, covering parts that are not normally conductive and can be immediately released from the patient. Examples would be operating theatre lighting, medical lasers, MRI scanners, hospital beds, and phototherapy equipment.
Type BF (body floating) covers equipment that comes into conductive contact with the patient, whether for a short or long time. It is probably the largest category of medical equipment and includes devices such as blood pressure monitors, non-cardiac surgical equipment, incubators, and ultrasound equipment.
The most stringent classification, Type CF (cardiac floating), is for applied parts that may come into direct contact with the patient's heart. A Type CF power supply provides a much higher degree of protection against electric shock and much tighter leakage current limits to the patient than a Type BF unit. This category includes medical devices for dialysis or ablation and electrocardiograph (ECG) machines.
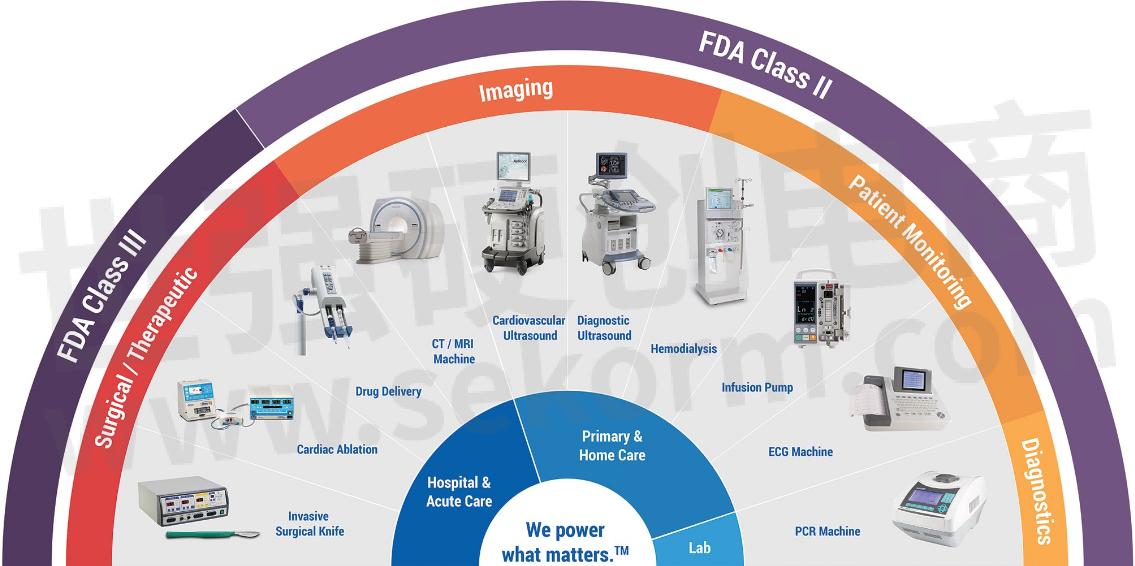
Fig.2
- +1 Like
- Add to Favorites
Recommend
- SL Power Announces New Power Supplies Optimizing The Convection Rating Performance
- SL Power‘s TREPP Optimization Enables Seamless Integration to Help Reduce Development Time and Cost
- SL Power‘ Power Supplies Expands Upon Targeted Applications Within Industrial Space
- The SLE Series Expands SL Power‘s Lean Line Ranging from 6 to 36 Watts
- SL Power Electronics, Global Supplier of Medical/Industrial Power Products, Offering AC/DC and DC/DC Power Conversion Solutions
- Agility EMS & SL Power to Offer New High-performance Internal Power Supplies and Desktop/wall-mount Power Supplies
- SL Power Electronics Solutions for Your Vertical Farms and Large Indoor Grow Centers
- Considerations When Selecting a Power Supply for Test Equipment
This document is provided by Sekorm Platform for VIP exclusive service. The copyright is owned by Sekorm. Without authorization, any medias, websites or individual are not allowed to reprint. When authorizing the reprint, the link of www.sekorm.com must be indicated.





























































































































































































































































































































































































































































































































































































































































































































































































































































































