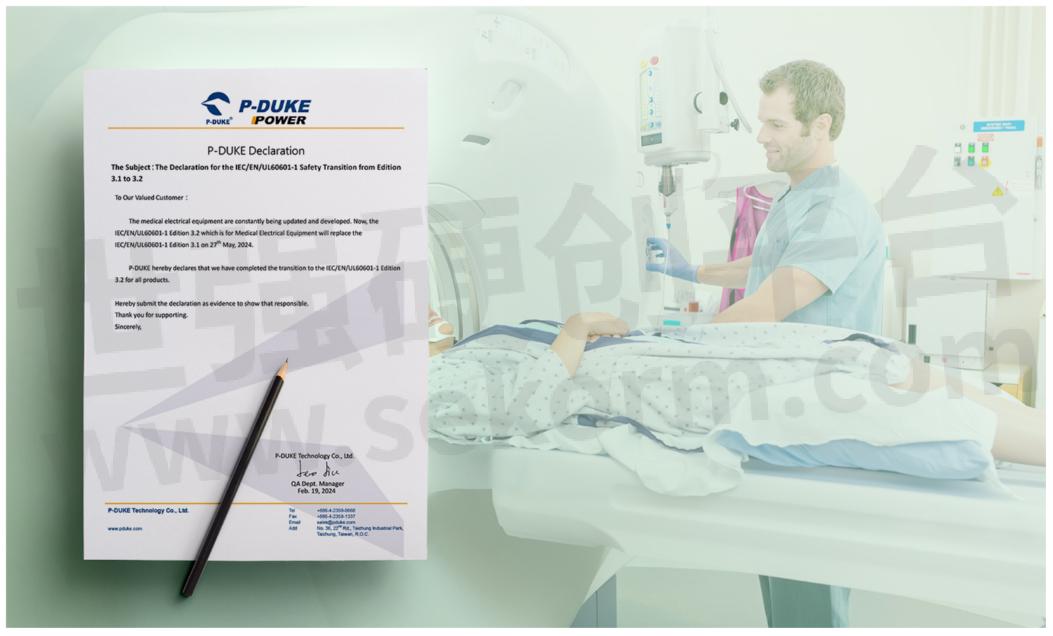
P-DUKE Latest Safety Standards Update: Edition 3.2 IC/EN/UL60601-1 Declaration

P-DUKE Declaration Update for the completion of P-DUKE transition to IC/EN/UL60601-1 Edition 3.2 for all products. Additionally, P-DUKE has upgraded P-DUKE IEC60601-1 certification from Edition 3.1 to the latest Edition 3.2 for medical products. This ensures that P-DUKE medical devices continue to meet and exceed the stringent safety requirements set forth by industry standards. Your continued support is invaluable, and we look forward to growing together in the coming years.
- +1 Like
- Add to Favorites
Recommend
- [Techno Frontier 2024 TOKYO] Visit P-DUKE !! East Hall No.3G-01
- P-DUKE Product EOL Announcement---DOS10-05T & DOH10-05T / DOS16-05T & DOH16-05T
- EOL Announcement--- P-DUKE LED15-xxS3P3 / LED15-xxS05
- Visit P-DUKE at the CMEF 2024
- P-DUKE launches the QAE and HAE ultra-wide input series DC-DC for supply interruption and change-over
- [Electronica 2024 Germany - München] VISIT P-DUKE!! Hall A4 No. 458
- Sep 24-27, 2024: Visit P-DUKE at InnoTrans Berlin – Hall 17, Booth 130
- UKCA (UK Conformity Assessed) Marking and their Impact on P-Duke Products
This document is provided by Sekorm Platform for VIP exclusive service. The copyright is owned by Sekorm. Without authorization, any medias, websites or individual are not allowed to reprint. When authorizing the reprint, the link of www.sekorm.com must be indicated.





























































































































































































































































































































































































































































































































































































































































































































































































































































































