Grepow Can Provide Integrated Power Supply Solutions For Prosthesis

The prosthetic limb is a replacement of missing body parts to help restore or obtain the fundamental function and mobility of the limb, mostly in the form of upper or lower limbs. In other words, the prosthetic limb mentioned here is not just physically able-bodied, but its function which needs power.

Prosthesis development and needs
With the improvement of science and technology, living standards rising, the intelligent prosthetic limbs that can be seen in the market now do not need to be embedded in the body but use human electrical tension of muscle to operate the prostheses. Just as our natural body is also driven by electromyography, the wearer can move the muscles based on brain wave signals. A consumer who experienced this technology showing a happy smile, said: wearing a prosthetic limb not only restored his original life but also played a positive role both physically and mentally on him!

Nowadays, smart prosthetic limbs have constantly improved in technology and materials (e. g., 3D printing technology or recycled plastic bottles), which help reduce costs, enhance durability, and easy replacement, while the spent materials are recycled. However, the demands remain excellent performance, safety, lightweight, suitable price, and sustainable operation.
A tip for battery selection
There is no denying that new energy batteries have also promoted the intelligent development of prosthetic limbs. How long a prosthetic limb can last and is light or not are important factors affecting the user experience. Lithium-polymer batteries dominate in shape and weight. Battery capacity can also be customized according to the demand. when choosing batteries, users and manufacturers should also pay attention to the thing as follows: does the battery selected by the prosthetic limb have safety certifications? It is not enough to meet the general requirements of the ISO9000 standards only, and It should also meet the certification requirements of ISO13485.
What is ISO13485?
ISO 13485 is one of the International Organization for Standardization (ISO) standards. It represents the requirements for a comprehensive quality management system for the design and manufacture of medical devices. In essence, ISO13485 certification is a voluntary management system certification, not for the product, but for the certification of the organization’s quality management ability. For enterprises, obtaining the certificate is more to prove the company’s strength and management level to customers, and increase the trust of customers and make them rest assured of consumption.
Grepow passed ISO13485 in 2020, which met the ISO13485's special requirements for the quality management system of ISO manufacturers.

About Grepow
Grepow Battery Ltd. has been committed to the research, development, and production of smart wearable batteries since 2013. We have the proprietary battery raw material formula of the battery industry and the core cutting-edge technology of lithium battery stacking. At the same time, it is also a frontier manufacturer of Li-Po batteries and shaped batteries in the large lithium battery industry.
So far, Grepow with more than 20-year experience in customer service has cooperated projects with a number of top 500 companies. Besides, It also has several successful cases on curved batteries. Tailored to the customers, a very perfect service system has been developed here to help us to understand the needs of intelligent prosthetic development better in a highly time, which saves way in the initial communication.
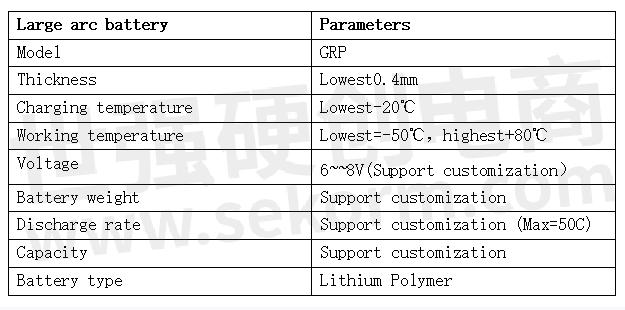
Grepow large arc(curved) battery model parameters

Seven reasons to choose Grepow for smart prosthetic products
1. OEM mass production, mature manufacturing process. Physical factory supply, quality assurance, and after-sales service;
2. High energy density, high output power, high capacity, and lightweight;
3. High battery consistency, conducive to series and parallel combination processing; can be combined according to different requirements;
4. Meet ROHS, SGS, CE, UL, etc.;
5. The battery pack installation method can be customized and sized according to the customers’ requirements. Such as shape, voltage, capacity, etc.;
6. Integrated power supply solutions can be provided;
7. Grepow passed the ISO13485 medical device quality management system certification and met the ISO13485 in 2020.
- +1 Like
- Add to Favorites
Recommend
This document is provided by Sekorm Platform for VIP exclusive service. The copyright is owned by Sekorm. Without authorization, any medias, websites or individual are not allowed to reprint. When authorizing the reprint, the link of www.sekorm.com must be indicated.

























































































































































































































































































































































































































































































































































































































































































































































































































































































































